A review
My first article for Science Based Medicine was a rebuttal to a paper published in The BMJ titled “Covid Vaccines for Children Should Not Get Emergency Use Authorization” by Drs. Wesley Pegden, Vinay Prasad, and Stefan Baral. Its central thesis was that “the likelihood of severe outcomes or death associated with COVID-19 infection is very low for children, undermining the appropriateness of an emergency use authorization for child COVID-19 vaccines”. Dr. Prasad further elaborated on Twitter by saying that,
Vast majority of kids recover quickly from SARS cov 2. And after all adults are vaccinated their risk of covid will decline precipitously. It’s ok to acknowledge that covid19 is not an emergency for kids.
I had multiple critiques of this article, namely that it completely glossed over how COVID-19 had harmed children, that it ignored the possibility of new variants, and that it didn’t mention the FDA had established stringent criteria for approving a vaccine under an Emergency Use Authorization (EUA). However, my core criticism was that the difference between an EUA and full FDA approval was nothing more than waiting several months after the pediatric trials concluded, something the BMJ article didn’t explain. Since most vaccine side effects emerge shortly after vaccination, I felt this waiting period alone would be of little value. As I wrote previously,
Their paper did not call for more or larger studies in children (which nobody would oppose), but rather a regulatory framework whose additional safety measure is waiting four additional months. In order for this delay to be of value, one would have to posit there is a significant vaccine side effect that is both unique to children and that it will only emerge between 2-6 months after vaccination at a high enough frequency that it will be detected under the limited structure of a clinical trial.
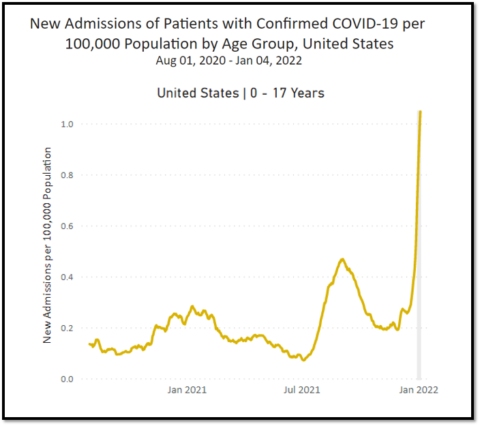
What’s changed: COVID-19
Since our respective articles were published in May 2021, new variants have more than doubled the pediatric death toll. The virus has killed over 550 American children since May, including healthy adolescents who were eligible to be vaccinated. Tens of thousands more children were hospitalized, some intubated in the ICU. Currently, more children are being hospitalized than ever before, almost all of them unvaccinated. 951 children with COVID-19 were admitted to the hospital on a single day this week, a huge number even factoring in incidental hospitalizations. Thousands more children contracted multisystem inflammatory syndrome in children (MIS-C), and most of these children need ICU-level care. 55 children have died from MIS-C.
What’s changed: The vaccine
Meanwhile, strong evidence emerged that the vaccine was extremely effective at preventing SARS-CoV-2 infections and keeping children alive and out of the hospital, a result that should have surprised no one. Five CDC studies examined the vaccine’s efficacy in adolescents. They found that:
- Study 1: In hospitalized adolescents, “179 COVID-19 case-patients, six (3%) were vaccinated and 173 (97%) were unvaccinated. Overall, 77 (43%) case-patients were admitted to an intensive care unit, and 29 (16%) critically ill case-patients received life support during hospitalization, including invasive mechanical ventilation, vasoactive infusions, or extracorporeal membrane oxygenation; two of these 29 critically ill patients (7%) died. All 77 case-patients admitted to the intensive care unit, all 29 critically ill case-patients, and both deaths occurred among unvaccinated case-patients.”
- Study 2: “Hospitalization rates were 10 times higher among unvaccinated than among fully vaccinated adolescents.”
- Study 3: “Among 272 vaccine-eligible (aged 12–17 years) patients hospitalized for COVID-19, one was fully vaccinated.”
- Study 4: Vaccine effectiveness “was 92% against SARS-CoV-2 infections irrespective of symptom status.”
- Study 5: “97/102 children with MIS-C were unvaccinated. None of the 5 vaccinated MIS-C patients required respiratory or cardiovascular life support (invasive mechanical ventilation, vasoactive infusions, or ECMO) compared to 38/97 unvaccinated MIS-C patients.”
Vaccine-related myocarditis emerged as a side effect in adolescent boys, but at a low-enough frequency (about 1 in 7,000) that only a massive trial that likely would have taken years to complete could have detected it. As this side effect occurs several days after vaccination, the waiting period proposed in the BMJ article would have been useless in detecting it. Moreover, researchers on vaccine-related myocarditis say that while long-term monitoring is needed, “most cases of suspected COVID-19 vaccine-related myocarditis in people younger than 21 are mild and resolve quickly”. Over 90% of adolescents and children with this condition have had their symptoms resolve, and the average hospital stay is two days. I know of no child in the entire world who has died from the vaccine, and tens of millions of children have been vaccinated in the USA alone. (The CDC is investigating one death of someone who was diagnosed with myocarditis after they died, but I do not know any details, including the age of the decedent.)
“The piece was not about vaccinating young kids”
With this in mind, it is interesting to see how the three authors of the BMJ paper have discussed it subsequently. On Twitter, Dr. Baral responded to critics by saying, “With all due respect, all we called for is the ACIP to meet and discuss. The piece was not about vaccinating young kids as compared to going through due diligence in deciding whether to vaccinate young children”. Dr. Pedgen agreed, saying, “The accusation doesn’t even seem to be that we argued against vaccinating kids (which we didn’t).” In an article for the Brownstone Institute, Dr. Prasad said,
In May of 2021, Wes Pegden, Stef Baral and I argued in the BMJ that kids vaccination should proceed via biological licensing agreement pathway and not the emergency use authorization. Because these risks were so low, we must demand robust evidence and large trials to show that the potential benefits of vaccination outweigh potential harms… We wanted large randomized trials.
If Dr. Prasad “wanted large randomized trials” at that time, why didn’t he say so in the BMJ article? As I previously stated, that request would have been hard to oppose.
Similarly, despite Dr. Baral’s subsequent claim that all his article called for was a meeting of the Advisory Committee on Immunization Practices (ACIP), his article did not even mention the ACIP. Of course, the ACIP was always going to meet and discuss the EUA for both adolescents and younger children.
Dr. Pedgen may be technically correct that his article did not explicitly argue “against vaccinating kids”. However, its entire point was to oppose the regulatory framework that was a necessary prerequisite to most children being vaccinated. So what’s the difference? It’s like telling my son he can use my car, refusing to give him the keys, and then feigning disbelief when he asks why I won’t let him drive. Dr. Mark W. Kline, a pediatrician who has seen children die of COVID-19, put it well when he responded to two of these authors by saying,
I’m sure you’re good at what you do, but the academic and semantic rationalizations you are offering up are typical of someone who doesn’t have to live with real-world consequences of their opinions and decisions. Just admit that you came to a flawed conclusion. No big deal.
If the BMJ article’s purpose was not to “argue against vaccinating kids,” what then was it arguing for, beyond empty platitudes such as “due diligence” and “robust evidence”? Moreover, even though the results had only been reported in a press release at that time, there was solid evidence that the adolescent vaccine trial was very successful. The press release stated “18 cases of COVID-19 were observed in the placebo group (n=1,129) versus none in the vaccinated group (n=1,131)” and the vaccine “was well tolerated”. Of course, the FDA had access to all this information and referenced it in their EUA announcement, noting that “the vaccine was 100% effective in preventing COVID-19” in adolescents.
I understand skepticism towards company press releases, but the existence of this trial was not even mentioned in the BMJ article. I believe that those who demand “robust evidence” should make some minimal effort to educate their readers about the evidence that currently exists. My article acknowledged this trial. My article acknowledged new variants could arrive that might significantly impact children. My article, unlike the BMJ article, also acknowledged that even before the arrival of these nasty variants, hundreds of children had died of COVID-19 and many thousands had been hospitalized. Overall, over 1,000 American children have died of COVID-19. You can read every article by some prolific writers on pediatric COVID-19 this pandemic and not learn this basic number.
The EUA was appropriate
The virus can really hurt some children, and the vaccine can prevent these rare, but grave harms. We have solid proof now of this now, but this was entirely predictable from what was known back then. It’s no surprise that the vast majority of deceased and hospitalized children since then have been unvaccinated. This is why I felt the EUA was appropriate for adolescents and younger children.
Currently, the COVID-19 vaccine is fully approved only for people 16 years and older. Pfizer filed for full FDA approval for children older than 12 years just last month, a process will likely take several months. Full FDA approval for younger children is many more months away. Children under 5 years cannot be vaccinated at all.
If regulators had adopted the policy proposed in the BMJ article, no EUA would have been issued for most children, and so few children younger than 16 years would be vaccinated today. If Drs. Pegden, Prasad, and Baral, don’t feel they came to a “flawed conclusion,” they should acknowledge this obvious fact, recognize its implications, and proudly defend it as a desired outcome instead of claiming an article titled “Covid Vaccines for Children Should Not Get Emergency Use Authorization” had nothing to do with vaccinating children.


